Family Practice Physician, Windrose Health Network, Hope, Indiana
The Effect of Introduction of Patient Reported Outcome (PRO) Measures by Implementation of Wireless Tablets on Depression Screening Rate for Adult Patients
Authors:
Hideki Tsunoda, MD; Samta Amin, MD, MA; Abigail Palmer, DO, MS MEd; Stephanie S. Richards, MD; Alissa Cohen, DO, MS, DipABLM; Gretchen Crum, LCSW; Stephanie Baverso, LCSW
Introduction:
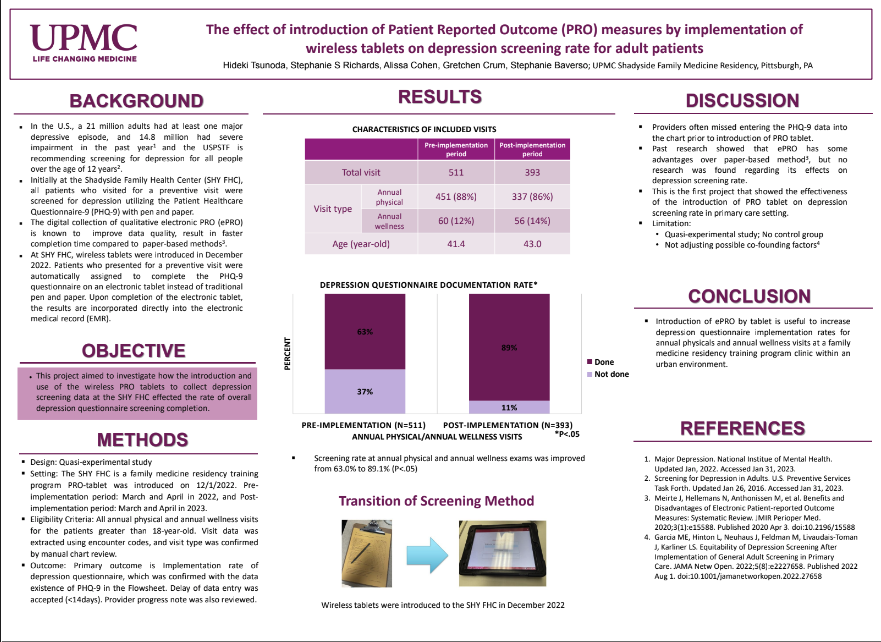
The U.S. Preventative Screening Task Force recommends screening all individuals starting at age12 for depression. At the UPMC Shadyside Family Health Center (SFHC), we screen all adult patients at annual and preventive visits for depression. Historically, the screening was done with a paper questionnaire, and providers entered the data into the electronic medical record (EMR)manually, which was a burden and was not completed consistently. Patient-reported outcome (PRO) measures were recorded electronically on a device such as a tablet is advantageous.
Methods:
Wireless tablets were first introduced at the SFHC in December 2022. Patients are given a wireless tablet to complete the depression screening questionnaire in the office instead of a paper questionnaire. Upon submission, the results are incorporated directly into the EMR. We reviewed all annual physical and wellness exam visits for patients more than 18-years-old March through April 2022 (pre-implementation period) and March through April 2023 (post-implementation period) and compared depression screening questionnaire documentation rates between the paper and PRO tablet screening conditions.
Results:
Depression screening questionnaire documentation rate was 322/511 (63.0%) with the paper questionnaire during the pre-implementation period and 350/393 (89.1%) with the PRO tablets during the post-implementation period (p<0.01).
Conclusion:
Introduction of wireless tablet significantly improved screening questionnaire documentation rate in the EMR.